Breast cancer is the most commonly occurring and most commonly studied cancer in the world
We see athletes and professionals donning pink; walks and talks; boxing gloves and ribbons; education and, ultimately, life-changing outcomes. But despite the progress and awareness surrounding breast cancer, there is still much to be done.
We looked back at the past 5 years of data on breast cancer research to better understand where we are, and more importantly, where we’re going. Our analysis leverages data available from Citeline’s Trialtrove® platform, which allows us to paint a clearer picture of the progress that’s been made.
Analyzing trends in breast cancer clinical trial data from the past 5 years
Year-over-year we see consistency in the number of trials started with a slight decline over the last several years. The greatest decline in breast cancer trials occurred in the Top 20 Pharma/Industry group of companies including Roche, Merck, AstraZeneca, Pfizer, Novartis, and BMS. Breast cancer trials sponsored by the Top 20 Pharma companies decreased from 138 to 87 from 2017 to 2021, respectively.
In addition, Academic sponsored trials decreased from 209 to 180 from 2017 to 2021, respectively. Interestingly, outside of the Top 20 Pharma and Academic trial sponsors, all other biotech and pharma industry-sponsored trials increased from 151 to 206 from 2017 to 2021, respectively.

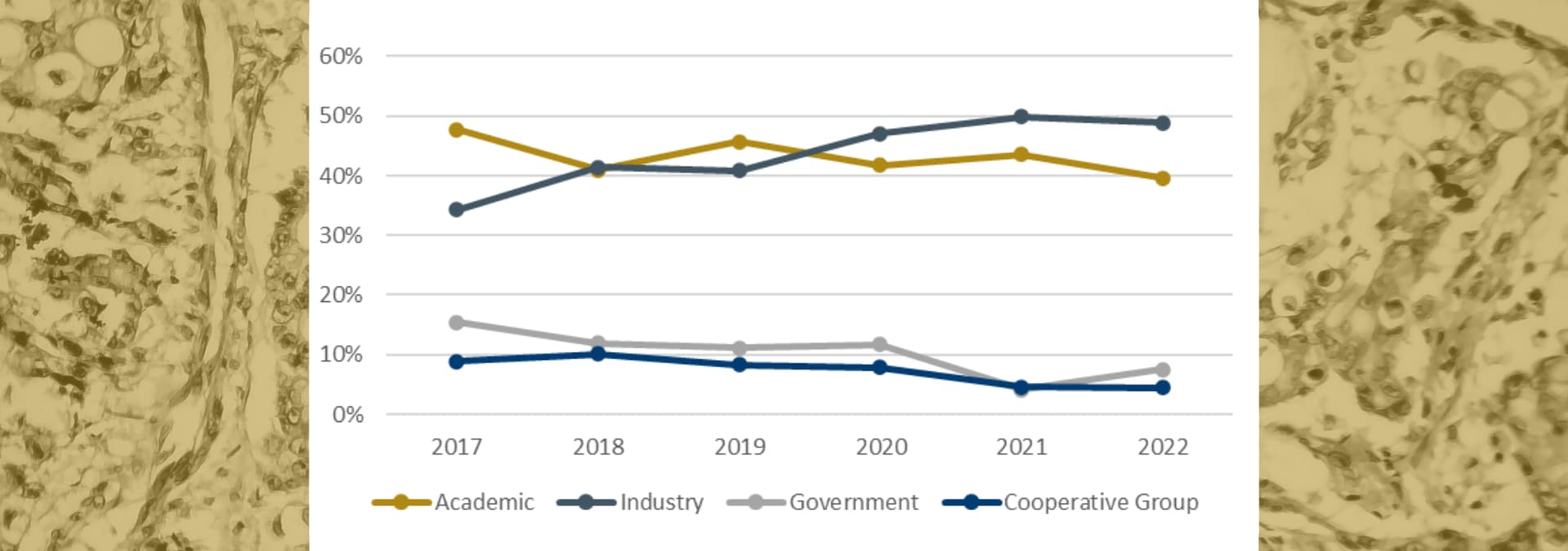
Who is sponsoring breast cancer research
Since 2017, the percentage of breast cancer clinical trials started by Academic, Government, and Cooperative Group sponsors has decreased. There are a few possible reasons for this, including changes in funding, a variety of resource-related challenges, or any number of issues related to COVID-19.
Where breast cancer research takes place
Regionally, the percentage of breast cancer trials has been consistent over the last 6 years in Asia, North America, and Australia/Oceania. In Europe, however, the percentage of active breast cancer trials is on a steady downtrend.

Phase trends of breast cancer clinical studies
In looking at the number of trials in Phases I, I/II, and II, we see a gradual decrease since 2017. The number of Phase III and IV trials increased from 2017 to 2021 with some year-to-year variability. These trial dynamics could imply that early-phase investigational agents are successfully moving through the clinical development cycle.

Research trends for breast cancer sub-types
Examining breast cancer subtypes Human Epidermal Growth Factor Receptor 2 (HER2+), Hormone Receptor positive (HR+), and Triple-Negative Breast Cancer (TNBC), we see the greatest number of clinical trials in patients with HR+ disease which also accounts for the greatest population of breast cancer patients (~70-80%).

Therapeutic classes of breast cancer research
The immuno-oncology (I-O) therapeutic class is the largest and broadest, accounting for 108 breast cancer clinical trials in 2021. The types of I-O therapies being tested in breast cancer include checkpoint inhibitors, targeting PD-(L)1, CTLA-4, CD47, ILT2, and Cbl-b; T-cell agonists, bispecific T-cell engagers, cell therapies including CAR-T, CAR-NK, CAR-M, and TILs; cytokine-based therapies; cancer vaccines, oncolytic viruses; and Toll-like receptor and STING agonists.

The number of breast cancer clinical trials studying antibody-drug conjugates
Antibody-drug conjugates (ADC) received a lot of attention in 2022, especially following data presented at ASCO from the DESTINY-Breast04 clinical trial that led to FDA approval of ENHERTU® (trastuzumab deruxtecan) in breast cancer patients previously characterized as HER2-negative. These data not only represent the success of another ADC therapeutic, but also the advances made in payload, linker, and targeting ADC technology.
Analysis of the number of ADC clinical trials in breast cancer by Phase shows that the ADC field is starting to mature going from predominantly early-phase clinical trials in 2017–2019 and transitioning to more Phase 2, registrational, and post-marketing trials since 2020.
With three ADCs approved to treat breast cancer patients currently and multiple other promising ADCs earlier in development, ADCs are a promising therapeutic class.

How you can further explore the data
We want to thank the dedicated team of analysts at Citeline, without whom this analysis could not have been completed. If you are interested in digging further into the analysis of the information covered above, you can do so by leveraging these links to the Trialtrove® research: HER2+, HR+, TNBC
- Please note that Citeline Trialtrove® is a paid platform, and a user subscription is required.
- Also, please be aware that Trialtrove® is a live and dynamic platform and that the data we shared as of 15 AUG 2022 changes on a real-time basis.
Navigating Breast Cancer Clinical Research & Commercialization
There has been tremendous progress made in the advancement of breast cancer therapies. As a purpose-built oncology CRO, Precision is uniquely positioned to support the demands of personalized medicine drug development, specifically in the areas of oncology and hematology.
Read more about the efficiencies of an end-to-end approach to oncology clinical research >




