Flow cytometry is a formidable technology for clinical research, but to be informative, it requires that the right sample be collected, processed, stored, shipped, and run according to specific processes and within defined time windows. In this article, we explore key considerations for selecting an appropriate matrix for flow cytometry assays used in clinical trials and share a selection of case studies where fixed blood was successfully used to generate meaningful study data.
Comparing Sample Types for Flow Cytometry Assays
Choosing the right sample for flow cytometry analysis depends on scientific, technical, and logistical factors. To select the optimal matrix, it is critical to consider what is being measured, which controls are appropriate, who will be processing and transporting the samples, and how much time will elapse between sample collection and analysis.
Fresh Untreated Whole Blood
Whole blood is the gold standard for receptor occupancy (RO) assays and is also used to perform absolute cell counting for establishing correlations between peripheral blood and tumor or evaluating depletions due to treatment. Many consider flow cytometry assays performed on whole blood to be closer to true physiology as all the different cell types are present in their in vivo ratios. Whole blood can also be drawn directly into vacutainer tubes containing virtually any stimulant, allowing flexibility in how a study is set up.
There can, however, be drawbacks to using fresh untreated whole blood. Time is a critical factor. For the most reproducible and representative results, flow cytometry needs to be performed within 24-48 hours post blood collection, which may not always be feasible from a logistical standpoint. Transportation of fresh untreated whole blood to lab testing sites may also require temperature regulation. If the blood is not well-mixed with an anticoagulant, clots may form and the cells within these clots—which may be cells of interest—will not be available for analysis. Further, certain cell types can be problematic. For example, neutrophils are less stable in fresh samples and activated T cells tend to die more quickly than naïve T cells.
Peripheral Blood Mononuclear Cells (PBMCs)
Isolated PBMCs can be frozen and then used for flow cytometry. PBMC isolation can be performed either at the clinical site, which can lead to variability, or at a central lab if the whole blood sample can be shipped same day or overnight. Freezing PBMCs comes with several advantages:
- Samples can be stored then analyzed after receiving clinical study results or at any convenient time
- Cohort or longitudinal analysis of samples can be performed in a single run, reducing variability on a per patient basis
- Cost to the sponsor can be reduced by batched analysis of samples
- Additional markers can be added after analysis of initial results, enabling modifications based on knowledge gained over the course of a trial
As with fresh untreated whole blood, PBMCs have their own set of drawbacks. The need for multiple sites to isolate PBMCs may add to cost, though this cost may be offset by batched analysis. With PBMCs, cell ratios are not preserved completely in comparison to whole blood. In addition, using PBMCs for RO assays may result in a loss of sensitivity because the drug may start to dissociate.
Fixed Whole Blood
Fortunately, for certain markers, the drawbacks associated with fresh untreated samples and PBMCs can be mitigated by stabilizing the whole blood example with fixatives. A key benefit of stabilizing samples is simplifying and streamlining operational execution, especially in global clinical trials. Since assays no longer need to be performed in real time, sample testing can be performed with one central flow cytometry lab, reducing cost and variability. This is particularly important for longitudinal samples, as with RO assays, and for markers that are sensitive to time or temperature in transit.
Choosing the Right Matrix for a Clinical Study
The intended application may narrow down sample choice (see Table 1). Fixed blood can be used for most assays, other than phospho-flow, keeping in mind that a fixative is tested to be compatible with the receptor, drug target, and the markers of interest in the panel.
Table 1. Appropriate sample choices for common flow cytometry assays
| Flow Cytometry Applications | Untreated Blood | PBMC | Fixed Blood |
|---|---|---|---|
| Immunophenotyping/Activation/Anergy | ✓ | ✓ | ✓ |
| Vaccines/Antigen Specific Tetramer/Peptide Flow | ✓ | ✓ | |
| Cell Therapy Flow (CAR-T, CAR-NK) | ✓ | ✓ | ✓ |
| PK Flow | ✓ | ✓ | |
| Receptor Occupancy | ✓ | ✓ | |
| Single Cell Sorting | ✓ | ✓ | |
| Intracellular/Intranuclear Staining* | ✓ | ✓ | |
| Phospho-flow | ✓ | ✓ |
Case Studies
Below we share three case studies highlighting Precision for Medicine’s experience in developing, validating, and utilizing fixed whole blood for flow cytometry assays in clinical trials.
Case Study #1: Monitoring target engagement for a first-in-class monoclonal antibody (MAb)
Precision for Medicine was tasked with developing an RO assay for monitoring target engagement and changes in receptor levels for a mAb targeting a co-stimulatory molecule on CD4 and CD8 T cells. Given the dispersed geography of the study, the 48-hour stability of the sample, and the limited time available for setting up the assay, it was determined that fixed blood would be the sample of choice. Feasibility studies were performed to identify the optimal fixative. While Cyto-Chex™ BCT tubes were not compatible with all markers in the panel, use of Smart Tube™ Proteomic fixative resulted in intra-assay, inter-assay, and inter-operator data that correlated with fresh untreated whole blood at three different drug concentrations. Testing demonstrated at least 120 days of stability at -80ºC, allowing for batched analysis. Based on these data, we developed and validated an RO assay and built a custom kit, complete with an aliquot of fixative, laboratory/site manual, and instructions on how to process and store the sample.
Case Study #2: Developing an RO assay for various cell types in blood and bone marrow
Precision for Medicine was asked to develop an assay for measuring RO on blasts, granulocytes, and stem cells in patients with acute myeloid leukemia (AML) for a clinical study with sites across the EU. Again, Smart Tube™ Proteomic fixative was added to both blood and bone marrow and a stability study determined marker stability up to 3 months enabling batch processing.
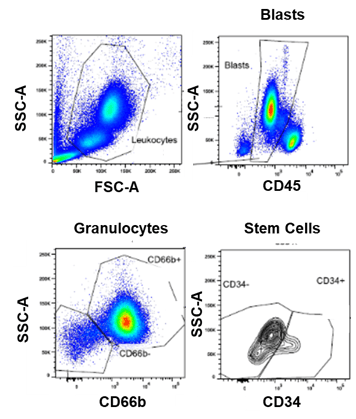
Figure 1. Representative RO analysis of fixed whole blood from a patient with AML using a 7-color flow cytometry panel
Case Study #3: Immunophenotyping for a global oncology study
Precision for Medicine developed a relatively complex, 15-color immunophenotyping panel for assessing T cell, NK cell, B cell, and memory cell subsets, as well as activation markers, in an oncology study with sites in Spain, Turkey, and Israel. Here, we used Cyto-Chex™ BCT tubes for direct patient blood draws. During assay development, we determined that the sample needed to be shipped at 4ºC and run within 96 hours to get the best resolution on the cell subsets. Thus, we worked closely with the sponsor’s clinical operations team to ensure that samples were packaged in an appropriate shipper and tested within the optimal time period.
Conclusion
Successful development, validation, and implementation of flow cytometry assays in clinical trials requires considerable forethought and careful planning. Choosing the right sample as early as possible—and understanding how it should be processed and when it should be run to get accurate, consistent results—is key. At Precision for Medicine, our decades of experience in flow cytometry enable us to develop creative, custom solutions that address the unique needs and challenges of each clinical trial, ensuring robust, reproducible data.
Learn more about Precision for Medicine’s comprehensive flow cytometry capabilities.




