An analysis of gene therapy clinical trial data collected over the past 5+ years
The success of gene therapies to date continues to spark an exciting second wave that is laying the foundation for next-generation technologies across several therapeutic areas—oncology, cardiovascular, muscular disorders, neurological conditions, and others.
To confirm our suspicions of an upward tick in the number of gene therapy programs initiated year over year, we turned to Citeline for a glimpse at the real figures.
- Please note: Though ex vivo genetically modified therapies (e.g., CAR-T, TCRs, oncolytic viruses, etc.) are classified as gene therapies by the FDA, they are not represented in the data analyzed in this article.
.webp?width=1024&height=360&name=Gene%20Therapy%20(1).webp) Gene Therapy Trials by Start Date
Gene Therapy Trials by Start DatePresent focus in early phase Gene Therapy
Not surprisingly, early phase research is where we see the majority of gene therapy trials. However, it is interesting to see that Phase I/II combination studies are the dominant early phase design choice for gene therapies. We recently wrote an article about best practices for executing Phase I/II combination studies, which may be helpful in your upcoming planning and considerations.
.webp?width=1024&height=360&name=Gene%20Therapy%20(2).webp) Gene Therapy Trials by Phase
Gene Therapy Trials by PhaseExamining the Current Pipeline of the Gene Therapy Trials
The gene therapy segment is busier than ever, with more than 120 open trials (Trialtrove® JAN 2022–AUG 2022). Citeline maintains a dedicated team of analysts who keep a keen eye on the number of planned clinical trials across the industry. Based on their tracking, we can see that Citeline Trialtrove® identified an additional 53 gene therapy trials on the horizon (see table below).
%20(1).webp?width=1024&height=360&name=Gene%20Therapy%20(1)%20(1).webp) Gene Therapy Trials by Status
Gene Therapy Trials by StatusHottest Therapeutic Areas with ongoing Gene Therapy Research
When diving deeper into the 128 presently open gene therapy clinical trials (as of AUG 2022), we see cardiovascular and CNS as the leading therapeutic areas with 26 clinical trials each (see table below). These are immediately followed by ophthalmology, oncology, and metabolic/endocrinology.
%20(1).webp?width=1024&height=360&name=Gene%20Therapy%20(2)%20(1).webp) Open Gene Therapy Trials by Therapeutic Area
Open Gene Therapy Trials by Therapeutic AreaTop 20 Diseases with Open Gene Therapy Clinical Trials
In examining the 128 presently open gene therapy clinical trials (as of AUG 2022), we gain a clearer picture of which diseases are drawing the most interest from sponsors (see table below).
- Hemostasis/Hemophilia leads with 13 currently open gene therapy clinical trials.
- Ophthalmology has a high concentration in two specific indications, macular degeneration and retinitis pigmentosa.
- Oncology features several tumor types with a few gene therapy trials in each of the major tumor groups.
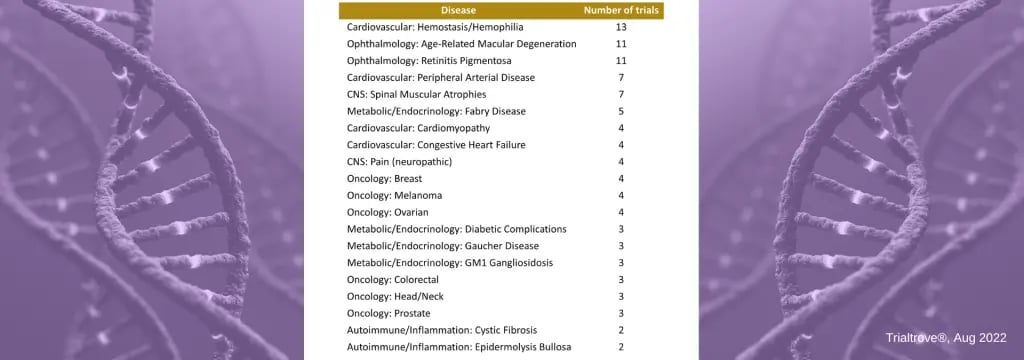 Open Gene Therapy Trials by Disease
Open Gene Therapy Trials by DiseaseTop 20 Countries with Open Gene Therapy Clinical Trials
The regulatory pathway for Gene Therapies has, of course, its unique nuances. We thought it worthwhile to pause and look at the top 20 countries with active gene therapy clinical trials. If you have access to Citeline, you can further investigate which trials and indications are active within each of the countries listed below.
.webp?width=1024&height=360&name=Gene%20Therapy%20Trends%20(1).webp) Open Gene Therapy Trials by Country
Open Gene Therapy Trials by CountryConclusion & Access to Citeline Data
In the future, genetic therapies may be used to prevent, treat, or cure certain inherited disorders, cancers, or infections. Promising early results and the potential of these therapies are driving the growth of this new category of personalized medicine. We want to thank the dedicated team of analysts at Citeline, without whom this analysis could not have been completed.
If you are interested in digging further into the analysis of the information covered above, you can do so by leveraging this link to the Trialtrove® research.
- Please note that Citeline Trialtrove® is a paid platform, and a user subscription is required.
- Also, please be aware that Trialtrove® is a live and dynamic platform and that the data we shared as of 15 AUG 2022 changes on a real-time basis.
Navigating Gene Therapy Clinical Research & Commercialization
The realization of next-generation cell and gene therapies calls for more specialized support. Through Precision Cell & Gene, your next project can take advantage of an intelligently designed combination of clinical, manufacturing, and commercialization solutions specific for cell and gene therapy development.
For more information on Precision Cell & Gene, contact us.




