Cross-functional, integrated data review preserves clinical research standards and data quality
During an oncology clinical trial, data may look different at different times and when viewed by different core team functional leads. To mitigate potential gaps that may exist in study data review processes, Precision for Medicine’s Clinical Science Analytics & Insights (CSAI) group applies a cross-functional, integrated data review solution to ensure the consistent application of oncology clinical research standards and to give sponsors a real-time scientific and clinical sense of the data. By standardizing oncology data review and review tools, CSAI helps core team functional leads manage data quality and supports critical on-trial decision-making.
Introduction to CSAI
The full power of clinical trial data can only be unlocked through the right approach to data analysis. CSAI is a dedicated team of oncology specialists with extensive solid tumor and hematologic indication expertise who understand the myriad factors that could potentially impact data quality throughout a study. CSAI achieves and maintains its objective of data quality by offering distinct, complementary services that apply clinical knowledge, relevant conceptual insight, thorough understanding of electronic case report form (eCRF) design and database builds, a risk management mindset, and a commitment to continuous improvement in oncology standards and operational processes.
As part of the cross-functional data review team, CSAI utilizes data visualization outputs customized to study objectives to support scientific remote data reviews, efficiently identifying and mitigating potentially systemic issues that can impact data quality (see Figure 1).
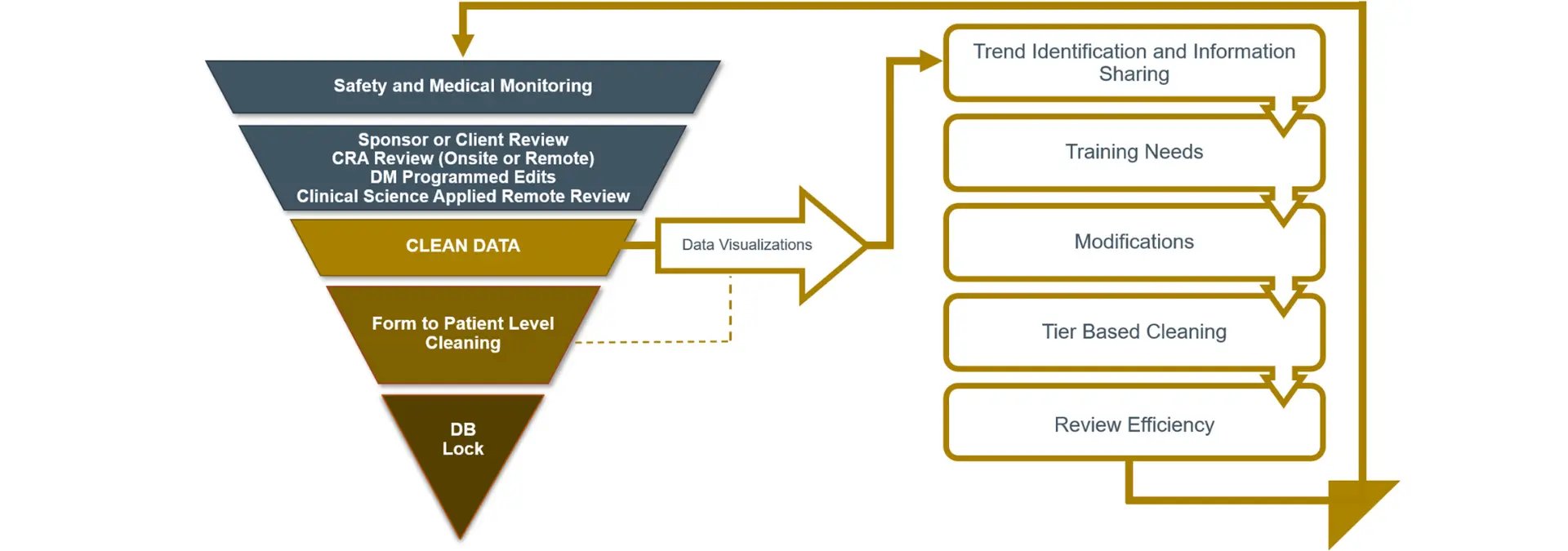 Figure 1. Optimizing data quality with an integrated review approach
Figure 1. Optimizing data quality with an integrated review approach
These data visualization outputs include proprietary Precision SMART Patient Profiles® (SPPs) that support holistic individual subject data review and All Patient Data Workbooks that facilitate aggregate review, data filtering, pivot tables, outlier and trend identification. Further, diagrams that enable high level process and role-based integrated data review drive collaborative quality outcomes (see Figure 2).
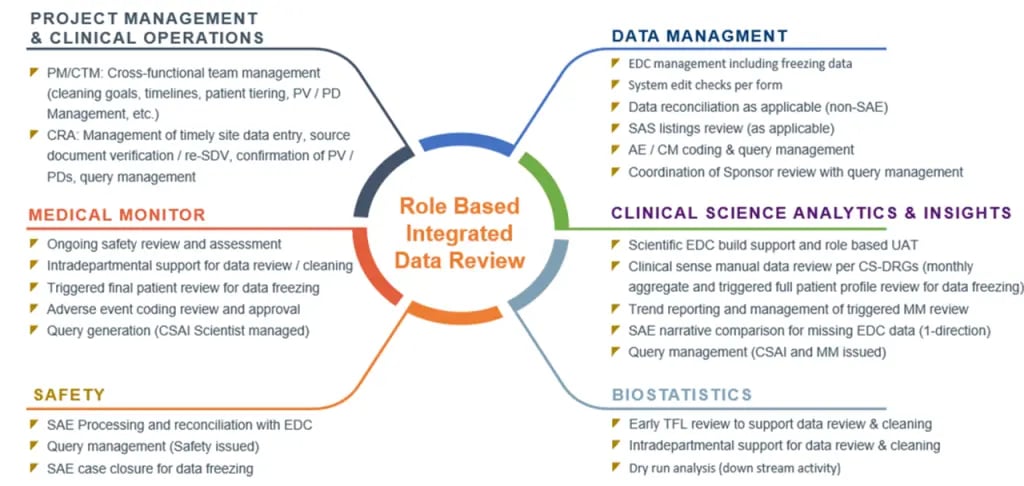 Figure 2. Core teams involved in cross-functional, integrated data review
Figure 2. Core teams involved in cross-functional, integrated data review
Each core team function represents a specialized discipline that is critical to the overall success of the clinical trial and data quality. CSAI’s exclusive data visualization tools integrate the various discipline-specific review outcomes to create an unprecedented level of data consistency.
Benefits of Including CSAI in an Oncology Clinical Trial
In addition to applying broad oncology indication expertise and technical data management acumen, CSAI leverages a range of study-specific tools to create data visualizations that can be used to drive on-trial decision-making, identify potential systemic errors, and trigger real-time corrective actions.
CSAI’s overall objective is to ensure the right review of the right data at the right time. Data cleaning milestones established at the start of a study trigger reviews performed by a CSAI scientist. These reviews may be augmented with periodic safety and efficacy targeted reviews and ad-hoc output runs and timed reviews as needed.
By facilitating cross-functional review and providing an additional layer of oversight, CSAI helps achieve and maintain data quality from first patient in (FPI) to database lock (DBL). CSAI also ensures consistent application and reporting of solid tumor and hematologic disease assessment criteria relevant to the indication and therapeutic under investigation.
Adding Value from Study Startup to Database Lock
CSAI provides support throughout the life of an oncology clinical trial. During study startup, CSAI:
- Reviews and offers input on the protocol, eCRF specifications, and indication-specific forms to ensure data collection and database structure are consistent with oncology clinical research standards
- Works with the sponsor to develop a Project Plan that includes cross-functional review expectations and outlines data collection technology, external data sources, run/review frequency and timing, outputs, data visualization tools, and study-specific reporting
- Creates Data Review Guidelines that define the manual review checks to be performed by each Clinical Scientist. This document is dynamic and updated as needed to address data issues or client requests
- Programs SPPs and other data visualization tools needed for the study
During enrollment, treatment, and follow-up, CSAI performs monthly aggregate and triggered full patient reviews, when appropriate. Any issues that arise during Clinical Scientist review are tracked and addressed with the appropriate study team member for resolution. CSAI also reviews the serious adverse event (SAE) narrative for potentially missing adverse events, concomitant medications, and procedures.
Prior to DBL, CSAI conducts a complete review of the full SPPs and triggers the final review by the Medical Monitor (MM). In our experience, the addition of CSAI to an oncology clinical trial significantly reduces both MM review time and the number of MM findings.
Key Takeaways
As oncology clinical trials grow increasingly complex, managing study data and ensuring data quality has become more challenging. By performing targeted reviews and analyses of clinical trial data throughout the life of a study, CSAI ensures data consistency, integrity, and accuracy with an emphasis on scientific and clinical sense, enabling continuous risk assessment and real-time use of data to support important decision-making and data quality.
Discover more ways Precision is transforming oncology research >




