Gene Therapy and Cellular Immunity
With two approved products currently on the market and a growing pipeline, adeno-associated virus (AAV)-based gene therapies have emerged as promising modalities for treating an array of hereditary diseases. AAV vectors offer several advantages as gene therapy vehicles, including high transduction efficiency, less immunogenicity than other viruses, and tropism for various target tissues.1 However, due to the viral origin of the vector capsid, these therapies using AAV vectors may elicit cell-mediated immune responses that destroy transduced target cells.
Further, pre-existing immunity may impact both efficacy and safety. An estimated 30-60% of adults carry AAV capsid-specific antibodies, with AAV5 antibodies having among the lowest prevalence.2 Adults may also have circulating capsid-specific CD8+ and CD4+ T cells due to natural exposure to AAV. Research has shown acquired immunity from the wild-type virus, coupled with innate immunity, can decrease the efficacy of AAV gene therapy vectors.3
For liver-tropic AAV vectors, there is some evidence that capsid-specific cellular immune responses, as detected by interferon-γ (IFN-γ) enzyme-linked immunospot (ELISpot) assays, can be associated with elevated plasma levels of liver enzymes.4 In some cases, elevated liver enzymes—particularly alanine transaminase (ALT)—were also associated with a decline in transgene expression, leading to a hypothesis that ALT is released into the plasma due to the targeting of transduced cells by cytolytic immune cells.5 Interestingly, increases in ALT can be mitigated using corticosteroids, which often also stabilize transgene expression.6 To date, though, there has been lack of consistency in correlating cellular immunity with elevated ALT levels or declines in transgene expression, underscoring the need for reliable, robust methods for immune monitoring.
A Method for Monitoring Cellular Immunity to AAV-based Gene Therapies
Researchers studying AAV-based gene therapies need methods for monitoring potential eliminatory T cell responses that may affect treatment outcomes. In a recent study, Precision for Medicine helped a sponsor optimize and validate a sensitive, robust, and reliable IFN-γ ELISpot assay. The assay was designed to monitor cell-mediated immunity against an AAV5-based vector encoding human B domain-deleted factor VIII (FVIII-SQ). ELISpot was selected as the method format because it offers a broad dynamic range and is both quantitative and sensitive to single-cell level. IFN-γ, a cytokine secreted by activated T cells and natural killer (NK) cells, was chosen as the marker for cell-mediated immunity.
Standardization of reagents and protocols is critical for establishing reproducible ELISpot results across multiple test sites and analysts.7 While regulatory guidance on ELISpot method validation is limited, standardized assay performance evaluations and acceptance criteria are essential for reliable data interpretation.8
Validating an IFN-γ ELISpot Assay for Monitoring Cell-Mediated Immune Responses
To validate certain performance parameters for this IFN-γ ELISpot assay, human peripheral blood mononuclear cells (PBMCs) were stimulated with peptides derived from AAV5 capsid proteins and FVIII. Due to the scarcity of AAV-5 and FVIII-specific human PMBCs, cytomegalovirus phosphoprotein 65 (CMVpp65) was used as a surrogate antigen for some validation parameters. The secondary benefit of using CMVpp65 is broader application of this method as a platform technology for future gene therapy products.
The key method performance parameters that were assessed and validated were:
- Limit of detection (LOD), which is used to distinguish positive responses from negative ones
- Confirmatory cutpoint (CCP), which was based on mock/antigen response ratio. The CCP would be used to adjudicate clinical trial samples in which both mock and antigen-specific response were at or above the LOD.
- Phytohemagglutinin-L (PHA) minimum response, which served as a quality control for PBMC functionality since PHA induces polyclonal IFN-γ secretion in T cells. The PHA minimum response limit was chosen to achieve an ~1% failure rate for the assay to align with the US Food and Drug Administration (FDA) immunogenicity assay guidance.9,10
- Precision, where intra-triplicate, intra-assay, and inter-assay precision were assessed by performing three assays runs, each with three donors, and interassay precision was assessed across nine runs. These validation assessments demonstrated that the ELISpot method had acceptable precision, but direct comparison of numerical results from different runs should consider potential inter-assay variability.10
- Linearity, which was confirmed by demonstrating that the number of responsive PBMCs plated was directly proportional to the number of spot-forming units (SFU) measured.10
- Range of quantitation between lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ), which defines where samples are quantifiable with acceptable precision.
- Robustness, which demonstrates that the assay has substantially the same performance under different experimental conditions.
- PBMC sample stability, which was tested by storing vials of donor PBMCs in liquid nitrogen over 18 months and periodically incubating the PBMCs under mock conditions or stimulating them with FVIII peptides to monitor response.
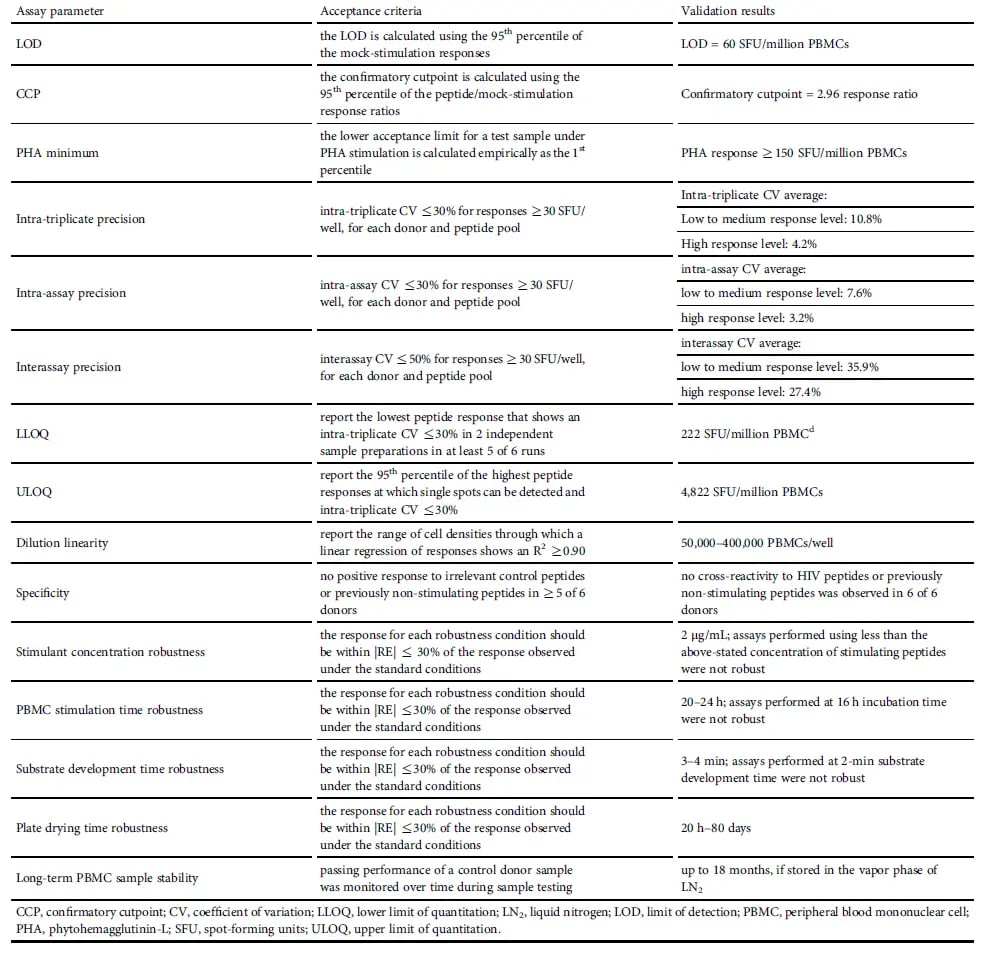 Table 1. Summary of IFN-γ ELISpot Assay Validation
Table 1. Summary of IFN-γ ELISpot Assay ValidationImplementing an IFN-γ ELISpot Assay in a Gene Therapy Clinical Trial
Once validated, the IFN-γ ELISpot assay was implemented in an AAV5-FVIII gene therapy clinical trial to monitor participants with hemophilia A for pre-existing and acquired cell-mediated immunity targeting the vector capsid or the expressed transgene. For control purposes, a donor who was not related to the study was also tested to ensure consistent assay performance.
Post-treatment cellular immune responses against the AAV5 capsid were observed in 94% of participants, beginning as early as two weeks following dose administration. Most AAV5-specific responses diminished over several weeks, eventually dropping below the limit of detection. At week 52, 93% of available samples had undetectable AAV5-specific responses by ELISpot. Responses against the transgene product were limited, with one participant who was positive at baseline and at week 52, and four participants who were positive only at single time points.10
Key Takeaways
ELISpot has been used to detect and monitor cell-mediated immunity in numerous therapeutic areas and applications, including AAV gene therapy trials where T cell responses may affect treatment outcomes.11–13 Here, an IFN-γ ELISpot method was validated against a set of standardized bioanalytical assessments to ensure reliable clinical data generation. Moreover, since ELISpot assays rely on the functionality of the stimulated cells, PBMC collection and cryopreservation were standardized to ensure the highest sample quality. Use of the validated IFN-γ ELISpot method in a clinical trial of AAV5-FVIII gene therapy demonstrated the utility of the assay for monitoring immune responses to viral vector capsids and expressed transgenes encoded by gene therapy vectors.
Explore validated methods for monitoring cellular immunity in AAV gene therapy trials >
References
1. Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: A long journey to successful gene transfer. Mol Ther. 2020;28;723-746.
2. Stanford S, et al. Adenovirus-associated antibodies in UK cohort of hemophilia patients: a seroprevalence study of the presence of adenovirus-associated virus vector-serotypes AAV5 and AAV8 neutralizing activity and antibodies in patients with hemophilia A. Res Pract Thromb Haemost. 2019;3:261–267.
3. Nidetz NF, et al. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol Ther. 2020;207:107453.
4. Mingozzi F, High KA. Overcoming the host immune response to adeno-associated virus gene delivery vectors: The race between clearance, tolerance, neutralization, and escape. Annu Rev Virol. 2017;4:511–534.
5. Nathwani AC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365.
6. Ertl HCJ, High KA. Impact of AAV capsid-specific T-cell responses on design and outcome of clinical gene transfer trials with recombinant adeno-associated viral vectors: An evolving controversy. Hum Gen Ther. 2017;28:328-337.
7. Britten CM, et al. T cell assays and MIATA: the essential minimum for maximum impact. Immunity. 2012;37:1-2.
8. Maecker HT, et al. Precision and linearity target for validation of an IFNgamma ELISPOT, cytokine flow cytometry, and tetramer assay using CMV peptides. BMC Immunol 2008;9:9.
9. Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, US Food and Drug Administration. Immunogenicity testing of therapeutic protein products — developing and validating assays for antidrug antibody detection, 2019. Available at https://www.fda.gov/regulatory-information/searchfda-guidance-documents/immunogenicity-testing-therapeutic-protein-productsdeveloping-and-validating-assays-anti-drug.
10. Patton KS, et al. Monitoring cell-mediated immune responses in AAV gene therapy clinical trials using a validated IFN-γ ELISpot method. Mol Ther Methods Clin Dev. 2021;22:183-195.
11. Dubey S, et al. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Imune Defic Syndr. 2007;45:20-27.
12. Körber N, Behrends U, Hapfelmeier A, Protzer U., Bauer T. Validation of an IFNg/IL2 FluoroSpot assay for clinical trial monitoring. J Transl Med. 2016;14:175.
13. Banas B, et al. Clinical validation of a novel enzyme-linked immunosorbent spot assay-based in vitro diagnostic assay to monitor cytomegalovirus-specific cell-mediated immunity in kidney transplants recipients: a multicenter, longitudinal, prospective, observational study. Transpl Int. 2018;31:436-450.




