The development of immunotherapy with checkpoint inhibitors in the last decade was a key breakthrough in the treatment of patients with various types of cancer. The efficacy of checkpoint inhibitors has been demonstrated for disseminated melanoma, renal cell carcinoma and non–small-cell lung cancer, suggesting that these therapeutics are a promising tool, especially for aggressive solid tumors.
While subgroups of patients treated with checkpoint inhibitors experience significant outcome improvements, generally only a part of the patient population responds and, in some cases, hyperprogression—accelerated tumor growth and progression following treatment—has been observed. To date, these patient subgroup-specific differences are poorly understood. Therefore, there is a significant clinical need for the development of reliable biomarkers that stratify patients and help to predict clinical response to cancer immunotherapy.
In a recent poster presentation at the 2022 ASCO® Annual Meeting, Epiontis ID immunophenotyping was used to characterize immune cells in the peripheral blood of patients with intermediate stage hepatocellular carcinoma (HCC) prior to treatment with transarterial chemoembolization (TACE, a localized chemotherapy) in combination with nivolumab, a checkpoint inhibitor.
Epiontis ID uses DNA-based epigenetic markers to characterize and quantify a wide range of immune cells in both peripheral blood and tissue. This technology harnesses the unique methylation patterns specific to distinct cell types and uses a customized process and quantitative polymerase chain reaction (qPCR) primers to amplify only those DNA regions that are demethylated in the cell type of interest, allowing for a precise count of those cells in any sample.
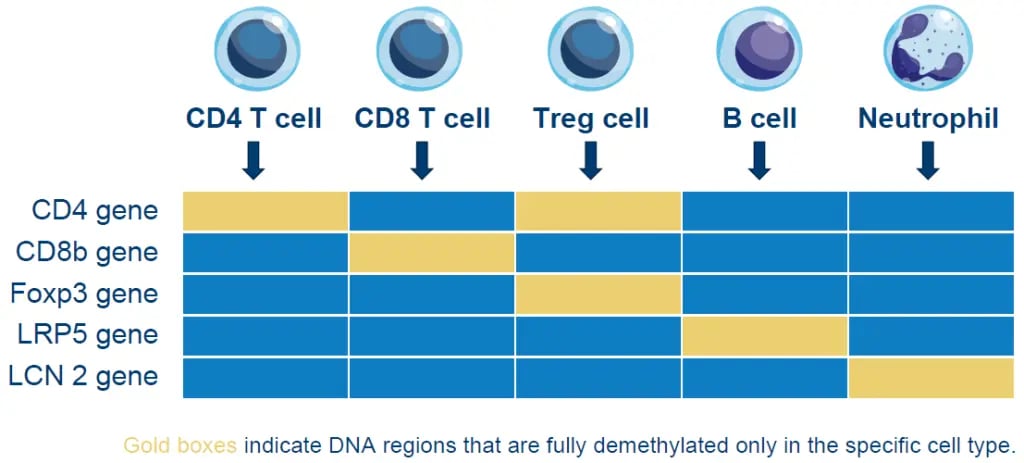 Figure 1. Epigenetic methylation for select immune cell types
Figure 1. Epigenetic methylation for select immune cell typesObjective
The primary objective of the study was to evaluate the safety and efficacy of TACE combined with nivolumab in patients with intermediate stage HCC and no prior systemic therapy. Secondarily, Epiontis ID was used to analyze pre-treatment whole blood samples for potential biomarkers or biomarker signatures associated with favorable clinical response.
Approach
Patients received up to two TACE treatments followed by nivolumab (240 milligrams every other week) initiated on Day 2-3 after the first TACE session and continued until progression for a maximum treatment duration of two years. Whole blood samples were collected pre-treatment from 39 of the 59 patients enrolled in the study. Epiontis ID was used to analyze these samples and quantify the numbers of three different immune cell types:
- CD8 T cells – Cytotoxic T cells involved in immune response, inter alia, against tumor cells
- Regulatory T cells (Tregs) – A key immune cell type for immune suppression
- PD1-positive cells – A marker that characterizes suppressed and exhausted immune cells
These immune cell counts were compared to those found in whole blood samples from healthy donors and evaluated for their potential to predict clinical response.
Results
The study met its primary endpoint, providing evidence for the efficacy of TACE in combination with nivolumab in patients with intermediate HCC and no prior systemic therapy. The results of the immunophenotyping demonstrated that the relationships among these three immune cell types, especially the ratio of Treg:CD8 T cells and the ratio of PD1-positive:CD8 T cells, show promise as a biomarker for the prediction of favorable clinical response in this patient cohort.
Specifically, patients with high Treg:CD8 T cell or PD1:CD8 T cell ratios experienced a significantly increased frequency of favorable clinical response, as defined by complete or partial response to treatment. In contrast, patients with low ratios of those two marker combinations—which each indicate a more active immune system—responded less favorably, as demonstrated by lower rates of response and higher rates of progressive disease.
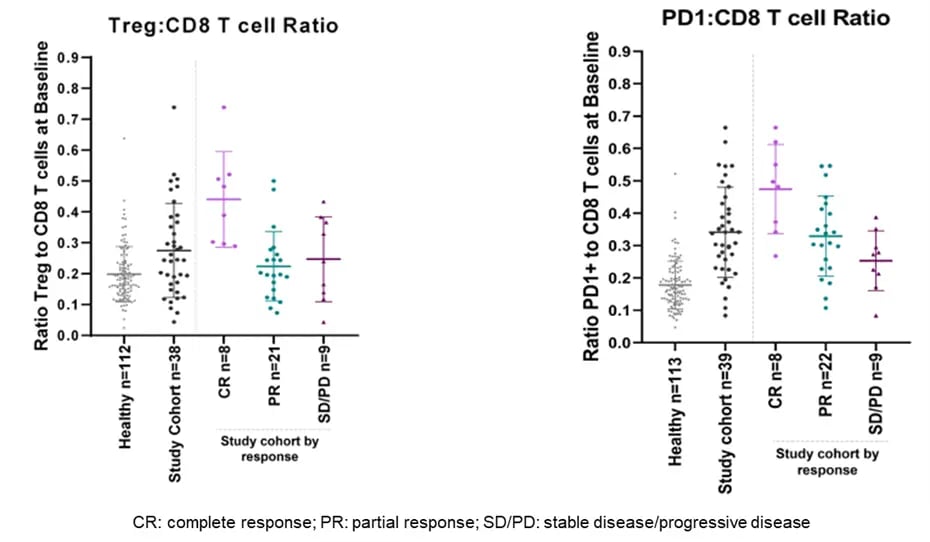 Figure 2. Immune cell ratios correlate with clinical response
Figure 2. Immune cell ratios correlate with clinical responseKey Takeaways
Pre-treatment Treg:CD8 T cell or PD1 positive:CD8 T cell ratios show potential as predictors of response to treatment with TACE in combination with nivolumab. Epiontis ID assays offer a promising tool for patient stratification that warrants further investigation of their potential use for the prediction of clinical response in patients treated with checkpoint inhibitor therapies.
While other immunophenotyping methods, notably flow cytometry, can also be employed for immune monitoring, Epiontis ID has a significant advantage: Results are highly reproducible and allow for comparison of results from separately sourced and frozen sample batches, which is crucial for reliable and comparable monitoring of patients at different times or in different studies.
Learn more about the applying epigenetic immunophenotyping to the prediction of clinical response in patients with intermediate stage HCC treated with TACE and nivolumab.