 Discover
Discover
false

Sallyanne Williams
Long-standing project management leader dedicated to improving delivery across all phases of product development. Working on a global scale, applies strategic smarts to operational delivery and financial management. Recognized as one of the PharmaVoice 100, some of the industry’s most inspiring people, awarded in the “Change Agent” category.
-
Read: Protocol Study Design: Bridging Clinical Goals with Patient Needs 
Protocol Study Design: Bridging Clinical Goals with Patient Needs
-
Read: Optimizing Immunohistochemistry Validation and Regulatory Strategies 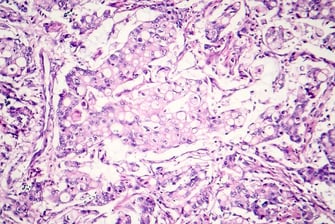 Discover
DiscoverTranslational Research - Biomarkers - Assays
Optimizing Immunohistochemistry Validation and Regulatory Strategies
| -
Read: Expediting Clinical Trial Study Start-up in Australia  Discover
DiscoverExpediting Clinical Trial Study Start-up in Australia
| -
Read: Rescuing a Complex Hematology Oncology Trial: Case Study in Precision  Discover
Discover
-
Read: Lean Authoring: Bringing Efficiency and Speed to Clinical Study Reports  Discover
Discover
-
Read: What Is an FDA Breakthrough Therapy Designation?  Discover
Discover
-
Read: Women Leaders Share Raw Truths About Leadership in Life Sciences  Discover
DiscoverClinical Trials - Translational Research
Women Leaders Share Raw Truths About Leadership in Life Sciences
| -
Read: Challenges & Opportunities in Cell and Gene Therapy  Discover
Discover
-
Read: How European Oncology Site Networks Streamline Clinical Trial Site Selection  Discover
DiscoverHow European Oncology Site Networks Streamline Clinical Trial Site Selection
|
Loading...
You've reach the end












