Looking Beyond 3+3 for Phase I Designs
Determining the Maximum Tolerated Dose (MTD) or Recommended Phase 2 Dose (RP2D) of a potential therapy is an essential early milestone which can set the tone for the entire clinical development program. Dose escalation methods for phase I cancer clinical trials fall into two broad classes—rule-based designs (which include the traditional 3+3 design and its variations) and model-based designs.
The 3+3 design and its variations use simple, prespecified rules to guide dose escalation. Due to its simplicity, the 3+3 design has been dominant in phase I clinical trials for decades despite some uncertainty about the true MTD and tendency to treat patients at low doses that are potentially sub-therapeutic.
More recently, model-based and model-assisted designs have grown in popularity due to their flexibility and effectiveness in early phase trials, often helping to correctly select the MTD and allocate more patients to the MTD.
-

Oncology - Clinical oncology - Clinical Trials - Early Phase Research - Clinical Trial Strategy
Phase I Clinical Trial Designs: Backfill i3+3 (Bi3+3)
- |
Introducing the Bayesian Optimal Interval Design (BOIN)
Novel designs provide practitioners with additional tools for conducting more flexible and efficient phase I trials. Bayesian Optimal Interval Design (BOIN) is one such model-assisted design that provides a novel platform to design phase I trials with a single agent, drug combination, and late-onset toxicity under a unified framework. It combines the simplicity of the algorithm-based design and the superiority of the model-based and can be implemented in an uncomplicated way.
Using the BOIN design, the observed DLT rate at the current dose is compared to pre-specified dose-escalation and de-escalation tolerances.
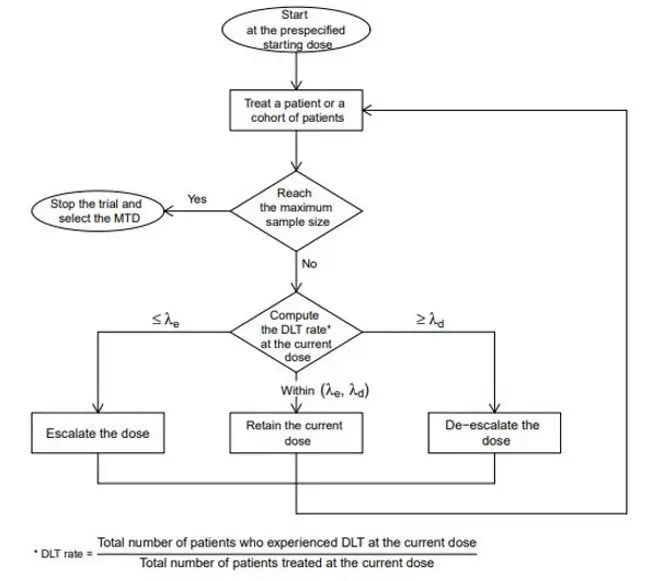
Considerations for the BOIN design in oncology clinical trials
What sets BOIN apart is the way it uses the observed dose-limiting toxicity (DLT) rate at the current dose to determine dose escalation and de-escalation. This approach is more transparent and assessable for non-statisticians and is simple to calibrate to fit a design goal.
Some have raised concerns regarding the possibility of aggressive dose escalation in BOIN trials, but studies show the BOIN design has a lower risk of overdosing patients than other designs and generally a higher probability of correctly selecting the MTD. More so, BOIN has been shown to rank among the top-performing designs for mitigating the risk of sub-therapeutic dosing, something possible with slow dose escalation designs.1
-

Oncology - Clinical Trials - Early Phase Research
Phase I Clinical Trial Designs: Modified Toxicity Probability Interval
- |
While the BOIN design does require some initial assumptions—including the target DLT rate (often 30%), cohort sizes >6 (up to a defined limit), and a maximum sample size—the accessibility of the BOIN design is enhanced by its simulation software.
With the assistance of experienced Biostatisticians, the user-friendly platform can assist in the design and implementation of the BOIN design in phase I clinical trials, including single-agent and drug-combination trials. Supporting the collation and analysis of data for on-going computations and long-term statistics, the BOIN design provides valuable insights that power clinical research.
Key takeaway
The BOIN design is a powerful and under-utilized tool for phase I oncology clinical trials. However, it is not a magic bullet—no model is. Precision’s data science experts can assist you in selecting the right design for your unique study requirements. This allows you to harvest the most impactful data to propel your project forward for further study.
Explore Precision Biostatistics >
Reference:
1. Zhou H, Yuan Y, Nie L. Accuracy, Safety, and Reliability of Novel Phase I Trial Designs. Clin Cancer Res. 2018;24(18):4357-4364. doi:10.1158/1078-0432.CCR-18-0168.


