What is the mTPI-2 study design?
The Modified Toxicity Probability Interval (mTPI-2) study design is a method used in early phase oncology clinical research for evaluating the safety and efficacy of investigational therapies. This approach, which is a variant of the traditional 3+3 design, includes key differences that make it particularly useful for certain types of clinical trials.
In this article, we will outline the key features of the mTPI-2 design and compare it to other commonly used study designs, such as the Bayesian Optimal Interval (BOIN) and the traditional 3+3 design.
-
Oncology - Clinical oncology - Clinical Trials - Early Phase Research - Clinical Trial Strategy - Clinical Data Management - Clinical Biostatistics
Phase I Clinical Trial Designs: Bayesian Optimal Interval Design (BOIN)
- |
Differences of mTPI-2 vs. BOIN and 3+3
One of the main features of the mTPI-2 design is its use of a modified toxicity probability interval (TPI) to guide dose escalation. Unlike the traditional 3+3 design, in which the dose is escalated in a predetermined manner until a predetermined maximum tolerated dose (MTD) is reached, the mTPI-2 design uses a modified TPI to guide the dose escalation process. This allows for more precise estimation of the MTD and reduces the risk of overtreatment.
Another key feature of the mTPI-2 design is its incorporation of Bayesian statistical methods. These methods allow for the incorporation of prior data and expert opinion into the analysis, which can lead to more accurate estimates of the MTD and improve the efficiency of the study. In contrast, the traditional 3+3 design does not incorporate prior data or expert opinion into the analysis.
The BOIN design is another commonly used method in oncology clinical research for evaluating the safety and efficacy of investigational therapies. Like the mTPI-2 design, it uses Bayesian statistical methods to guide the dose escalation process and incorporate prior data and expert opinion into the analysis. However, the BOIN design differs from the mTPI-2 design in its use of a Bayesian optimal interval to guide the dose escalation process, rather than a modified TPI.
-

Oncology - Clinical oncology - Clinical Trials - Early Phase Research - Clinical Trial Strategy
Phase I Clinical Trial Designs: Backfill i3+3 (Bi3+3)
- |
Advantages of mTPI-2 in Phase I Oncology Clinical Trials
The mTPI-2 design can be implemented similar to the traditional 3+3 design. The advantages are that it is more flexible than the 3+3 and it also possesses superior operating characteristics that are comparable to more complex model-based designs, such as the continual reassessment method (CRM). This design provides an upgrade to the modified toxicity probability interval design, with a substantially lower risk of overdosing and a better precision to identify the MTD.
The mTPI-2 study design offers several advantages in oncology clinical research. Its use of a modified TPI to guide dose escalation and incorporation of Bayesian statistical methods can provide more precise estimates of the MTD, reduce the risk of overtreatment, and improve the efficiency of the study. This makes it particularly useful for certain types of clinical trials, such as those with investigational therapies that carry a significant risk of toxicity.
mTPI vs. mTPI-2 (example)
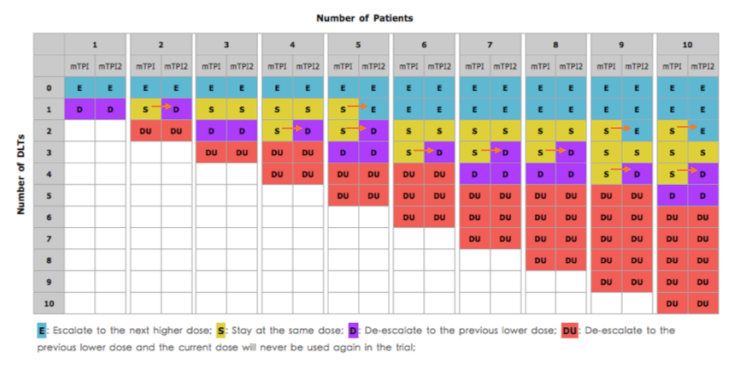
Summary of mTPI-2
The mTPI-2 study design is a viable option for evaluating the safety and efficacy of investigational therapies. It provides a rigorous approach to data collection and analysis, which allows for a detailed assessment of potential toxicities and side effects.
Additionally, the use of a modified TPI and the incorporation of Bayesian statistical methods in the mTPI-2 study design can provide more precise estimates of the MTD, reduce the risk of overtreatment, and improve the efficiency of the study. It’s tailored for researchers and practitioners in the healthcare industry, but also for patient education in clinical trial process and the effort to improve cancer treatment.