CASE STUDY
Biomarker Assay Validation for Intra-Tumoral Quantification of Phosphorylated VEGFR-2
It remains unclear whether angiogenesis inhibitors, e.g., Avastin, have an effect on the entire tumor vasculature or specific endothelial cell sub-types. Variation in endothelial marker expression may be related to differences in differentiation or functional status, and play a role in resistance to drug targeting.
Scientists at ApoCell have developed innovative biomarker assays to measure the intra-tumoral effects of VEGFR-2 inhibitors on VEGFR-2 phosphorylation (p-VEGFR-2) and downstream biological consequences of the drug-target interaction. Results from this study revealed that VEGFR inhibitors preferentially target discrete populations of tumor endothelial cells associated with the smaller peripheral blood vessels. Furthermore, the assay allowed localization of the drug effects within the entire cross.
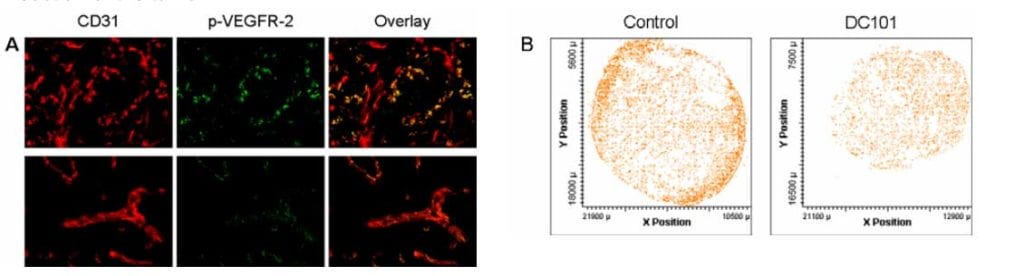
A, Laser generated immunofluorescent images of CD31 (red), p-VEGFR-2 (green), and overlay images showing co-localization of endothelial cells positive for p-VEGFR-2 from controls (top panel) and DC101-treated tumors (bottom panel). Note that DC101 treatment decreased p-VEGFR-2 compared with controls.
B, Tumor sections stained in A were subsequently scanned by LSC to generate an X and Y coordinate position of endothelial cells positive for either p-VEGFR-2 (top row) or total VEGFR-2 (not shown)
within whole tumor cross-sections. Note that constitutive expression of p-VEGFR-2 appears most intense at the periphery of untreated tumors and decreases after DC101 treatment as indicated by the pixel density.
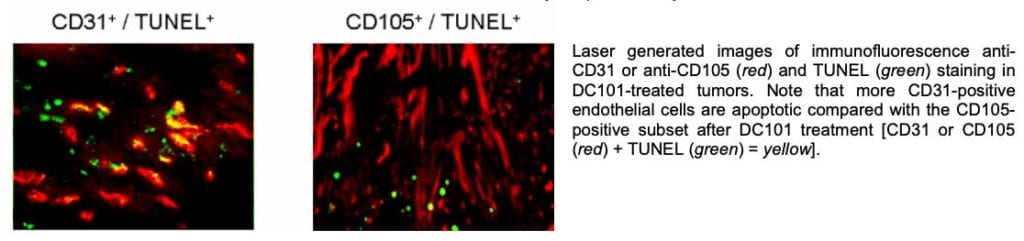
These biomarker assays were developed to directly assess whether an agent (DC101) intended for the VEGFR-2 can target all the tumor vasculature. Using sophisticated protein target mapping, it was demonstrated that the effects of DC101 are localized to specific areas within the tumor. Specifically, DC101 decreased overall tumor micro-vessel density, but it mostly affected smaller CD105-negative micro-vessels located in the periphery of the tumor. Intriguingly, anti-VEGFR-2 therapy resulted in increased mean vessel size and an increase in overall VEGFR-2 levels. Increases in total VEGFR-2 levels were localized to the tumor core and were associated with increased expression of the oxygen-sensitive transcription factor, hypoxia inducible factor-1α. These data suggest that VEGFR inhibitors preferentially target discrete populations of tumor endothelial cells associated with the smaller peripheral blood vessels. Thus, agents that target a single receptor (e.g., VEGFR-2) may not be sufficient to completely inhibit tumor angiogenesis.
This assay may be used to evaluate candidate compounds early in pre-clinical studies to determine which drugs are most biologically active. Additionally, drug delivery and/or cellular penetration can be measured using the sophisticated tissue mapping procedure.
References
1. Cancer Res. 2004 Jul 1;64(13):4601-10