CASE STUDY
Pharmacodynamic Analysis of DNA Damage Biomarkers in CTCs as Part of the Phase III BEACON Trial in Breast Cancer®
Clinical Application: Pharmacodynamic analysis of a DNA damaging agent to identify a predictive signature for clinical response
Key Words: CTCs, ApoStream®, pharmacodynamics, DNA damage, protein expression, LSC, breast cancer
Background: Topoisomerase 1 is a nuclear enzyme that plays an essential role in DNA replication, transcription, recombination, and repair. It is a common drug target in oncology. One such therapeutic agent, etirinotecan pegol (NKTR-102), is a unique long-acting topoisomerase 1 inhibitor designed for prolonged tumor cell exposure; its active metabolite, SN38, stabilizes the topoisomerase 1 complex, subsequently resulting in double-strand DNA breaks and eventual apoptosis.
The Phase 3 open-label, randomized, multicenter BEACON study compares NKTR-102 with treatment of physician’s choice (TPC) in patients with locally recurrent or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine, and examines the pharmacodynamic effects on DNA damage biomarkers upon treatment with NKTR-102.
Methods: ApoCell first developed and qualified a multiplexed biomarker assay for detection of six different biomarkers – Topoisomerases 1 and 2, γH2AX (a marker of double-strand DNA damage), RAD51 (a member of double-strand DNA damage
repair machinery), Ki-67 (proliferation marker), and ABCG2 (efflux transporter for SN38) – in CTCs by Laser Scanning Cytometry (LSC).
Blood was then collected from all patients enrolled in the study at four time points, as illustrated in Figure 1.
All specimens underwent CTC enrichment by ApoStream®; enriched cells were cytospun to slides and stained with the six biomarkers of interest and CTC phenotype markers (cytokeratin, CD45, and DAPI).
CTCs were identified as DAPI+/CK+/CD45- cells, and the expression level of each marker was examined in these cells. Additionally, apoptosis was measured in the isolated CTCs using TUNEL.
Results:

Figure 1. Treatment and Biomarker Analysis Schedule. CTCs were drawn at C1D1, C2D1, C4D1 and End of Treatment for analysis of target-specific biomarkers in CTCs
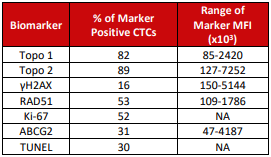
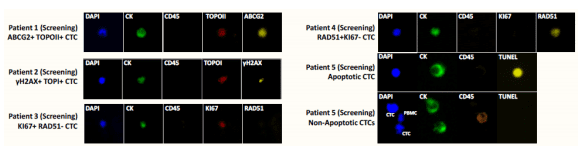
C1D1 Samples. CTCs were detected in 93% of pre-dose samples (median: 217; range: 7-15000 cells). Biomarkers were expressed in varying ranges and intensities across all specimens.
Impact: ApoCell has successfully designed and qualified an assay for simultaneous detection of multiple DNA damage biomarkers in response to therapy, and has successfully
incorporated this assay into a Phase III clinical study. The BEACON study indicates that ApoStream® is able to isolate CTCs from a majority of patients, and represents a lessinvasive means of obtaining biologically significant data to inform treatment decisions. Pharmacodynamic biomarkers can be reliably measured in CTCs, and can be a potential
predictive measure of clinical response.
References: 1. Hoch U, et al. “Etirinotecan Pegol Target-Specific Pharmacodynamic Biomarkers Measured in Circulating Tumor Cells (CTCs) ApoCell, Inc. from Patients in the Phase III BEACON Study in Patients with Metastatic Breast Cancer (mBC).” ASCO 2014.