CASE STUDY
Validation of an LSC Method for Measurement of γH2AX in Human Peripheral Blood Mononuclear Cells
Clinical Application: Monitoring the extent of DNA damage in response to chemotherapy
Key Words: assay development, γH2AX, DNA damage, PBMC
Background: Many chemotherapeutic agents are designed to inhibit growth or induce apoptosis in malignant cells by inducing DNA damage. Topotecan, an inhibitor of DNA isomerases, intercalates between DNA bases and causes double strand breaks (DSBs), and the absence of DNA repair leads to apoptotic cell death. Phosphorylation of histone H2AX on serine 129 (γH2AX) occurs at sites flanking DNA DSBs and can provide a measure of the number of DSBs within a cell. To monitor the extent of DNA damage and ultimately the efficacy of topotecan or similar agents, ApoCell was developed a biomarker assay for the detection of the expression levels of γH2AX.
Methods: Blood from healthy donors was treated ex vivo with topotecan. Several cellular subtypes were examined (CD3+, CD4+, CD14+, CD15+, CD16b+, CD19+, CD20+, CD33+, CD34+, CD45+, CD133+) using Laser Scanning Cytometry (LSC) to identify the subpopulations showing the greatest induction of γH2AX in response to treatment. The three subtypes that exhibited the highest induction of γH2AX were then further analyzed to determine a dose-response relationship. The assay was transferred to another facility, where training and cross-validation was performed to demonstrate the reproducibility of the results.
Results: The initial screening of cellular phenotypes showed mixed results in γH2AX induction in response to topotecan treatment. The phenotypes that exhibited the most consistent responses were CD3+, CD14+, and CD16b+. These three phenotypes were further examined in two distinct donors after two hour treatment at two concentrations of topotecan as shown in Figure 1 below. Cross-calibration of the LSC instruments at both test sites was completed to determine equivalent scan settings, and then the effects of treatment with a DNA damaging agent on a cell line was compared at both locations, as seen in Figure 2.
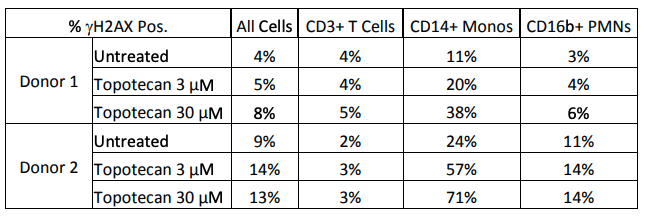
Figure 1. Effects of topotecan treatment on γH2AX+ cell counts. The effects of treatment with topotecan were examined in three cell phenotypes in two donors. Two hour treatment at the above concentrations did not induce an increase in CD3+ T cells, CD16b+ PMNs, or the population of all PBMCs when viewed as a whole; however, a clear dose-dependent increase was observed in both donors in CD14+ monocytes.
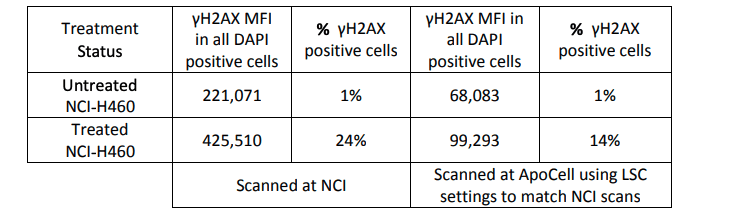
Figure 2. Results of LSC Method Cross-Validation at ApoCell and NCI. After optimizing scan settings between the two sites, NCI-H460 cells were treated with gemcitabine, another DNA damaging agent. Treated cells were compared to untreated cells at both sites in both the strength of γH2AX staining and the percentage of positive cells. The trend at both locations illustrates an induction of γH2AX after treatment.
Impact: ApoCell has developed a reliable assay for the detection of γH2AX in different cellular subtypes from human blood. The assay was successfully transferred to other laboratories.
References: 1. Davis, D.W., et al. “Sensitive Detection of Gamma-H2AX Induction as a Pharmacodynamic Marker for Profiling Patients Treated with Topotecan.” ASCO, 2010.