

Central Lab
Services
Precision medicine and biomarker-driven clinical trials pose a set of challenges unique from classical clinical trials, and require unique central lab services solutions.
The complexity inherent in kit development, logistics, sample management, and data requires an approach to central lab services that is systematic, robust, and can simplify clinical trial management: a translational central lab services solution. Precision for Medicine offers central lab services in support of global trials including kitting, logistics, sample processing, biostorage, and related biospecimen data services. In addition, Precision offers a comprehensive suite of specialty lab services including flow cytometry, genomics and NGS, bioanalysis, and tissue and liquid biopsy analysis.
Central Lab Services designed for biomarker-driven clinical trials
Precision’s harmonized approach is rooted in a deep understanding of the needs of complex biomarker trials—where excellence in customized kit development, logistics, data management, and technology are necessary.
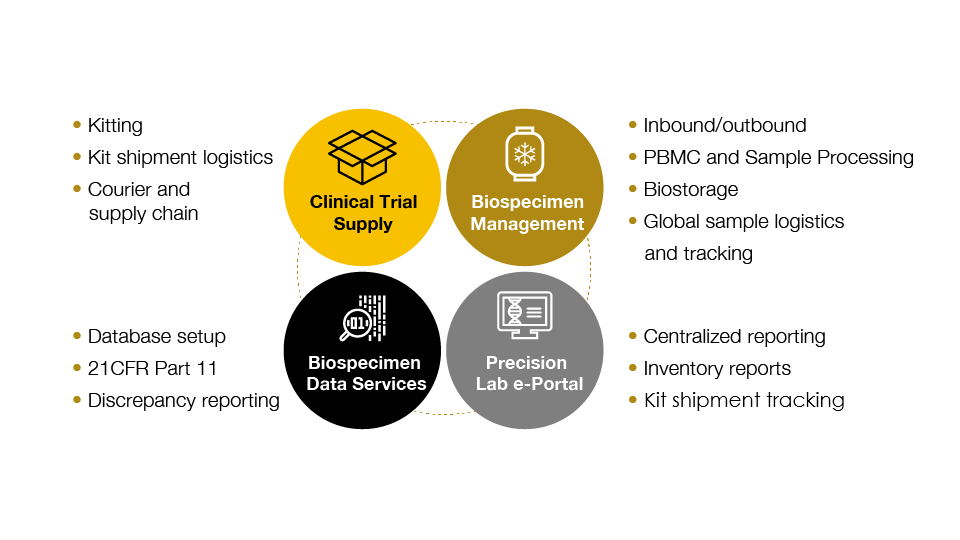
Advanced program management drives rapid project startup and study success
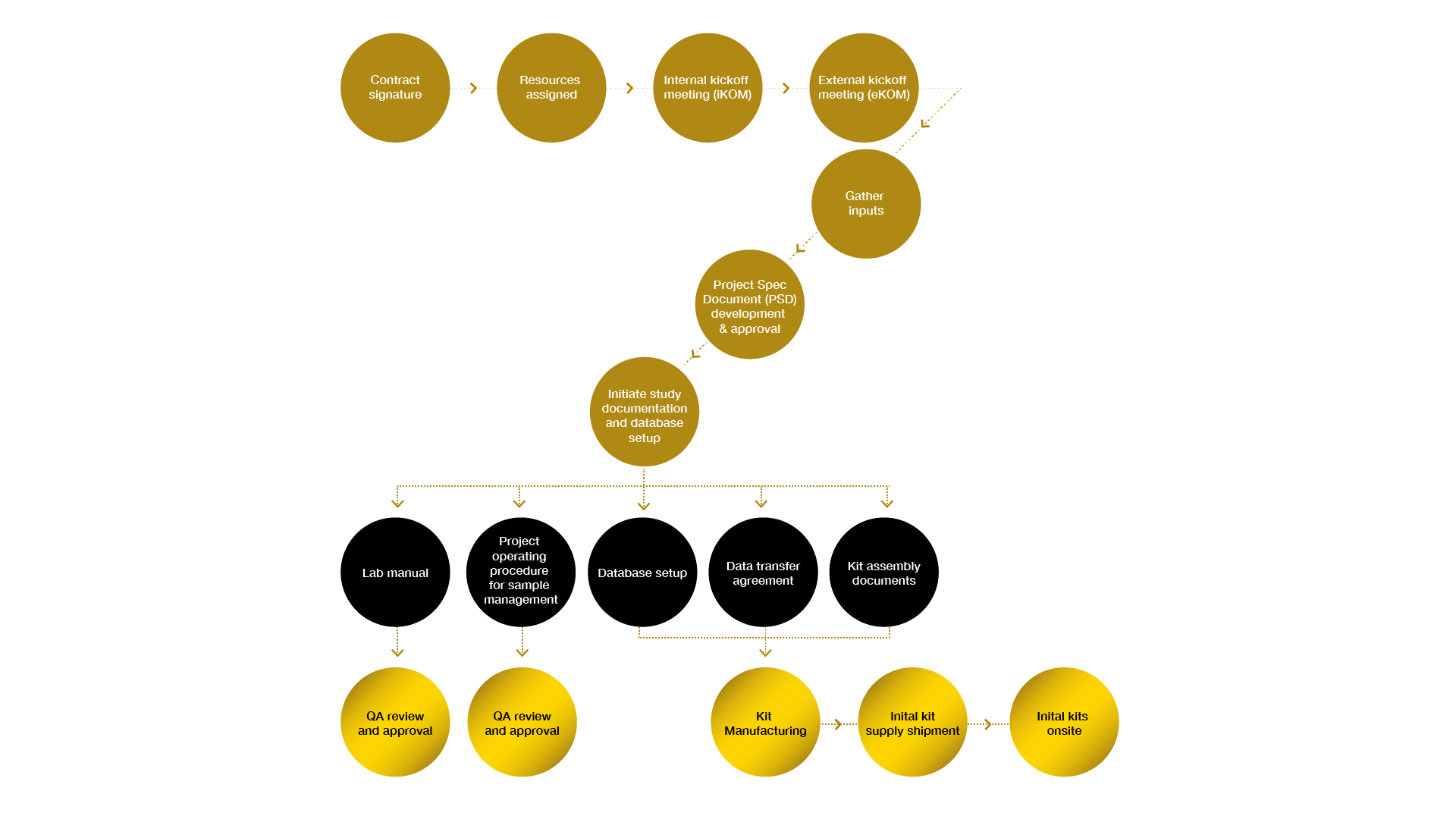
A clear program management structure is critical in order to consistently execute on all aspects of a central lab services project. Precision’s approach integrates all aspects of a central lab service project with a single point of contact and provides:
- Dedicated study startup structure
- Document creation
- QA-approved lab manual creation
- Site training documentation
- Designated project manager as the point of contact
Well-structured and coordinated processes for project initiation promote a successful startup with rapid study startup timelines.
Project initiation workflow
Expansive global reach ensures timely and consistent sample processing and analysis
Clinical Reach
Translational Central Lab Services: Solving the complex challenges of biomarker-driven clinical trials






/AdobeStock_320736962.jpeg?width=343&height=396&name=AdobeStock_320736962.jpeg)
