
Immune Monitoring by Precision Epiontis ID®
Epiontis ID® is unique in its approach to clinical immune monitoring
- Simplified Logistics
- Wide Range of Sample Matrices
- Proven Clinical Utility
- Precise and Reproducible Results
-
Easy Logistics,
Reliable ResultsEpiontis ID® is versatile and compatible with diverse sample types, with minimal sample processing required. Samples can be transported ambient within a 3-day window or batch-shipped in a frozen state. All assays are uniformly performed in facilities accredited with ISO 17025 and compliant with GCP/ICH guidelines.-
Choose from >35 fully validated assays
-
Collect sample at clinical site without processing
-
Ship ambient or frozen in batches
-

Samples arrive at central lab, measured with quick turnaround
-
-
Wide Range of Sample Matrices
Monitor immune cells in whole blood, tissue, and many other biological samples:- Dried blood spots (DBS)
- Peripheral blood mononuclear cells (PBMC)
- In vitro expanded cells
- Whole blood (heparin, EDTA, Paxgene vials)
- Clotted blood from serum tubes
- Formalin-fixed paraffin-embedded (FFPE) samples
- Fixed blood (lysed erythrocytes)
- Fresh-frozen tissue
- Tissue stored in RNAlater
- Urine

-
Proven Clinical Utility
Explore resourcesEpiontis has been used routinely in clinical studies with over 100,000 clinical samples analyzed, including as a secondary outcome measure.
This has produced data routinely included in peer-reviewed publications and poster presentations at conferences such as AACR, ASCO, and AAI.

-
Precise and Reproducible Results
Excellent comparability of results within and across studies allows for highly flexible batch selection, adding additional cell types and samples to a study as needed.- Fully automated process and use of references and calibrators ensure highly reliable results
- Long-term monitoring shows absence of shifts or drifts
- This allows flexible batch selection: Measure a subset first, then extend number of subjects or assays

Epiontis ID® explained
Combining the Power of Epigenetics and the Consistency of qPCR, Epiontis ID® harnesses the unique methylation patterns specific to distinct cell types and uses a customized process and qPCR primers to amplify only those DNA regions that are demethylated in the cell type of interest.
The quantitative nature of qPCR then allows for a precise count of the number of cells of interest in any sample. The uniformity and objectivity of qPCR results yield highly reproducible data that can be compared within and across various studies.
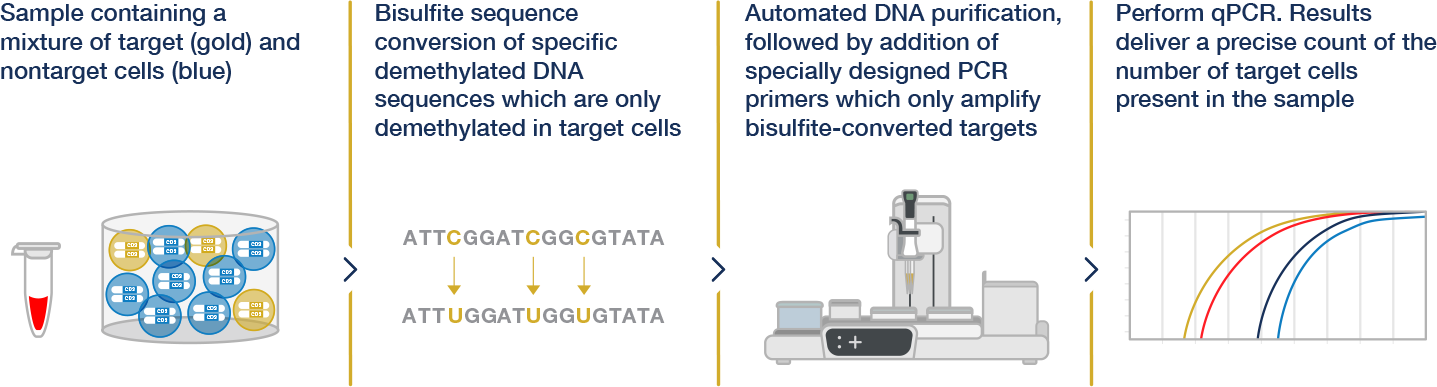
See how Epiontis ID® technology works
Within our suite of immune monitoring services, Epiontis ID® proprietary technology stands out by offering an epigenetic approach to cell profiling. Watch to learn how.
Precision Epiontis ID® :
Use Cases Across Diverse Therapeutic Areas
Epiontis ID® has many uses across therapeutic areas; this is a collection of the myriad examples of how Epiontis ID® can be used in advanced research.
All of Epiontis ID®’s assays are fully validated and can be rapidly set up to run
Download the full list of validated panelsThe scientific team behind Epiontis ID® continuously adds new available cell types, often in response to specific client requests or needs. We do more than just create a customized assay; we provide a comprehensive biomarker strategy that incorporates Epiontis ID® and various other technologies to help achieve your clinical development objectives. Currently, there are over 35 validated assays available for Epiontis ID®, with additional cell types added regularly. Study sponsors can select and combine any of the available cell types into a panel for analysis.
| T Lymphocytes | Other Immune Cells | Exhaustion / Activation / Migration Markers | Other Cells types (Fibrocytes) |
|---|---|---|---|
|
|
|
|