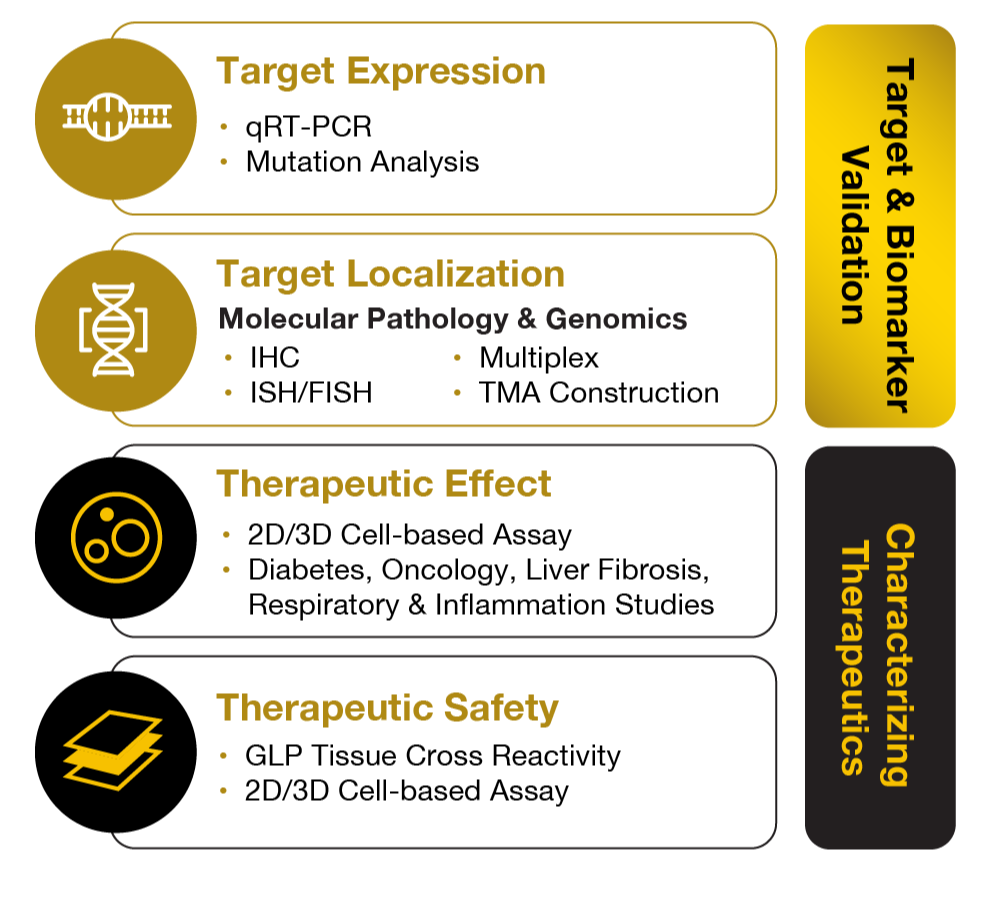
Developing a Comprehensive Strategy for Implementing AI & Multi-Omics for Translational Research
|
-

Kaylee Mueller
has not third author: true, (SizeLimitingPyMap: {main={hs_id=199448088864, hs_child_table_id=0, hs_updated_at=1762810484868, hs_published_at=1771396615394, avatar=Image{width=1200,height=1200,url='https://5014803.fs1.hubspotusercontent-na1.net/hubfs/5014803/Kaylee%20Mueller.png',altText='Kaylee Mueller',fileId=199456336149}, lastname=Mueller, hs_initial_published_at=1762810487482, hs_created_by_user_id=26433386, hs_created_at=1762810467146, hs_is_edited=false, hs_deleted_at=0, name=Kaylee, slug=kaylee-mueller, hs_updated_by_user_id=65160865}, second={}, third={}})